Com.Pl.it DX® Lung
Molecular Testing for Non-Small Cell Lung Carcinoma (NSCLC)
The ComPlit DX® multigene test provides important information on tumor biology and can lead to the identification of carcinogenic mutations known as ‘Driver Mutations’. As a result, this information helps the treating physician and the patient to reach the most effective personalized treatment for Non-Small Cell Lung Cancer (NSCLC).
Selecting a Suitable Treatment Strategy for Patients with Non-Small Cell Lung Cancer (NSCLC)
The ComPlit DX ® Lung multigene test is specifically formulated for patients with Non-Small Cell Lung Cancer (NSCLC) and may also be applicable to other solid tumors, including those of unknown original origin.
The Com.Pl.it DX® Lung Cancer Assay:
ascertains the molecular profile (gene mutations) of the tumor and the relationships among genes in instances of numerous mutations.
identify approved medications that target either the mutant gene(s) or the associated pathways.
discerns mutations linked to resistance against targeted medicines.
Suggests therapies sanctioned for the particular mutation, although applicable to a different tumor type (off-label), and/or identifies medicines already undergoing clinical studies.
Genetic Panel
Twenty-seven gene mutations
- AKT1, ALK, BRAF, CDKN2A, CTNNB1, DDR2
- EGFR, ERBB2, FBXW7, FGFR1, FGFR2, FGFR3
- HRAS, KEAP, KRAS, MAP2K1, MET, NOTCH1
- NRAS, PIK3CA, POLE, PTEN, RET, SMAD4
- PTEN, SMAD4, SMARCA4, STK11, TP53
Genetic Panel
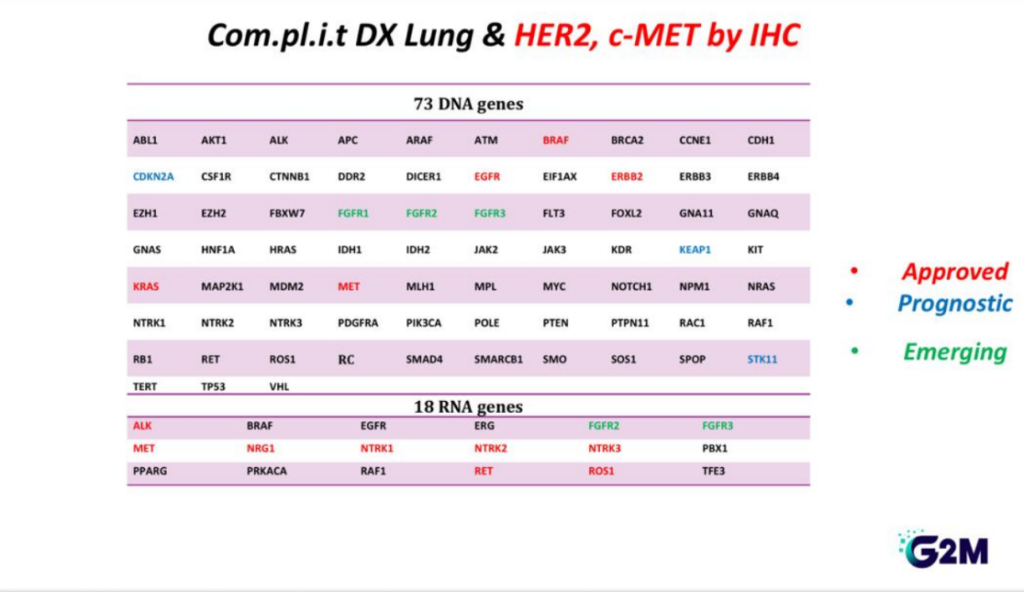
Seven gene rearrangements
- ALK, MET, NTRK1, NTRK2, NTRK3, RET, ROS1
- The FGFR1-3 are now the emerging bio markers for NSCLC patients and they can be benefit from targeting therapy as well as for the rare group of patients with NRG1mutation
Biomarkers for Immunotherapy
- PDL- 1
Immunohistochemical Biomarkers
- HER2
Common Inquiries
It is intended for those diagnosed with Non-Small Cell Lung Cancer (NSCLC). It may also be applicable to various forms of solid tumors, including those with an unidentified primary origin.
Results for the Com.Pl.i.t DX® Lung Assay will be accessible within 15 business days.
For Com.Pl.i.t.DX® Lung analysis, we require either the paraffin block from the tumor or uncolored paraffin sections mounted on slides (air-dried, not oven-dried). We require four portions of 3μm and six parts of 10μm.
The specimen must be maintained at ambient temperature (25°C). We advise including an ice pack in the bag during the summer season (The ice pack must not come into direct contact with the samples).
Your results will be transmitted to your doctor through a secure network and to you via email.
sci-pharm Medical SA holds certifications for ISO 9001:2015 (Cert. No. 041150049) and ELOT ISO/IEC 27001:2013 (Cert. No. 048190009) from TUV NORD HELLAS, dubai Health Authorities 2024 ,necessitating written agreement from each patient for the utilization of their genetic material for diagnostic purposes
It is also mandated by data protection regulations.
To finalize the test, you must complete and submit the Consent form available at the link below.